"I'm glad to tell you that Comirnaty from today has received approval for children five to 11 years of age," said Marco Cavaleri, head of vaccine strategy at the European Medicines Agency (EMA), using the vaccine's brand name.
"This is based on a different dose in the one used in adults, essentially it's a much lower dose," he told an online public meeting.
The vaccine was already cleared for use in people aged 12 and over in the 27-nation EU.
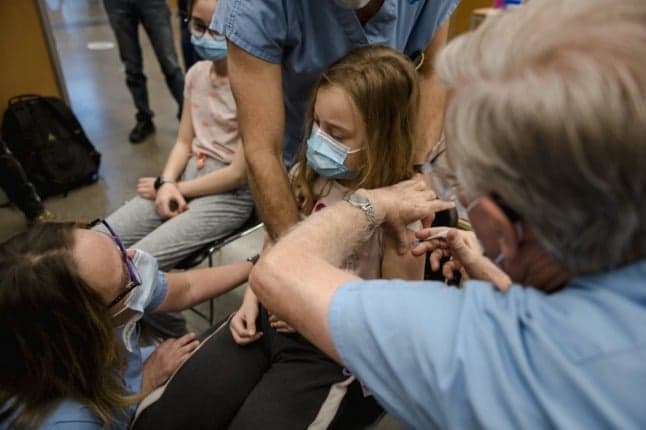
Children aged five to 11 will be given one third of the dose that older people receive, with two injections, three weeks apart, the EMA said in a statement.
The vaccine was 90.7 percent effective in a study of nearly 2,000 children of that age, it added.
Side effects were usually "mild or moderate" lasting a few days, and included pain in the injection site, tiredness, headache, muscle pain and chills.
The EMA "therefore concluded that the benefits of Comirnaty in children aged five to 11 outweigh the risks, particularly in those with conditions that increase the risk of severe Covid-19."
The EU Commission will now likely approve the vaccine for children aged 5 to 11 but the ultimate decision over whether to roll out the Covid jab to yound kids will rest on the government of each member state.
‼️ EMA recommends approval of BioNTech/Pfizer’s #COVID19vaccine, Comirnaty, for children aged 5 to 11.
— EU Medicines Agency (@EMA_News) November 25, 2021
In this population, the dose of #Comirnaty will be lower than that used in people aged 12 and above.
Read the full press release: https://t.co/uqqBAklVvl pic.twitter.com/NZQhli4SDl
France on Thursday said ministers were examining rolling out the vaccine to the age group but said there would be no decision before 2022.
But the Pfizer jab's safety in children "will continue to be monitored closely".
Health authorities say children make up an increasing proportion of new cases and hospitalisations in Europe, which is back at the centre of the coronavirus pandemic.
Children are also considered key drivers of infections even when they themselves do not come down with symptoms.
In the Netherlands, where the EMA is based, authorities said earlier this week that the largest increase in cases was among children up to the age of 12.
"We know that severe Covid-19 and death remain quite rare in children, however disease of all severity occurs in all the paediatric ages," Cavaleri said.
"Moreover, high transmission results in increased hospitalisation in children of all ages."
While children with underlying health conditions were more likely to become ill, the majority of children in hospital with Covid were otherwise healthy, said Cavaleri.
They were also at risk of so-called "long Covid" symptoms dragging on for months after infection, and multisystem inflammatory syndrome, he added.
The EMA is separately reviewing Moderna's coronavirus vaccine for children aged 6-11 and expects to reach a decision in January.
The regulator has so far approved four vaccines for use for adults in the EU: Pfizer and Moderna, which use messenger RNA technology, and AstraZeneca and Johnson & Johnson, which use viral vector technology.
Join the conversation in our comments section below. Share your own views and experience and if you have a question or suggestion for our journalists then email us at [email protected].
Please keep comments civil, constructive and on topic – and make sure to read our terms of use before getting involved.
Please log in here to leave a comment.